1. Course introduction
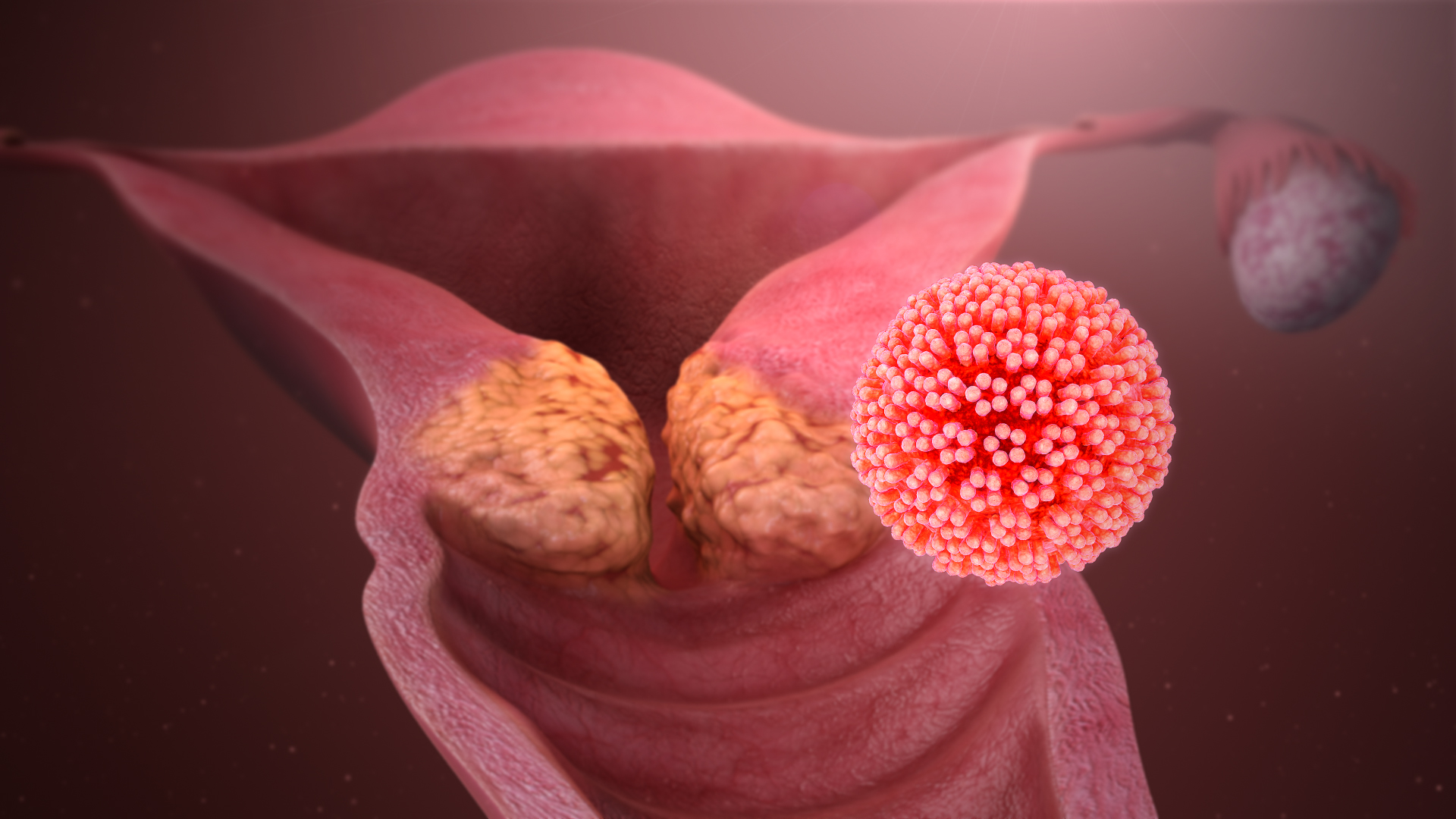
Cervical cancer is one of the most common cancers in women. The main culprit of cervical cancer is the HPV virus, found in 99% of cervical tumors. Therefore, periodic HPV screening is of great significance for the early diagnosis of cervical cancer. The course “HPV GENOTYPING SCREENING FOR CERVICAL CANCER USING REALTIME PCR” is to provide students with in-depth knowledge and hands-on practice throughout the course. Students will learn about the technical principles and new technological solutions in HPV genotyping screening, can proficiently perform HPV screening procedures, analyze results, and solve problems during the testing process.
2. Training method
- Team-based learning: encourage critical thinking, creativity, and proactive learning through group discussions, and group presentations
- On-the-job training: Students observe and practice alongside the lecturers
- Teaching equipment: A 300 m2 Lab with modern equipment from many well-known manufacturers around the world
3. Course objectives
- Comprehend the structure, classification, epidemiology, and transmission route of the HPV virus
- Comprehend genome structure and pathogenic mechanism of HPV
- Comprehend the principles of Realtime PCR using PNA Probe technology in HPV genotyping screening
- Proficiently perform the sample processing and DNA extraction procedure using different methods
- Proficiently perform Realtime PCR using PNA Probe technology in HPV genotyping screening
- Comprehend causes and solutions to common errors during the process
4. Syllabus
- Overview of HPV virus: the structure, classification, epidemiology, transmission route.
- Molecular mechanisms of HPV-induced Carcinogenesis and methods of detecting and typing HPV virus.
- Realtime PCR using PNA Probe technology in HPV genotyping screening
- Practicing DNA extraction using different methods.
- Perform Real-time PCR reaction and melting curve analysis to provide counselling and inform test results.
- Summary. Q&A. Certificate awarding ceremony.
5. Course information
- Course Code: TN - 01
- Subjects: Doctors, bachelors or engineers specialized in testing in healthcare facilities; bachelors of Biology, Biotechnology, Chemistry
- Training time: 03 days (8 lessons/day)
- Number of students: 12 - 16 students per class
- Course opening: Open every month upon the number of applications
- Registration package:
- 01 Application form of Phacogen Institute of Technology HERE
- 01 notarized copy of university or college diploma
- 01 notarized copy of identity card or citizen identification